Structural Biology
Overview
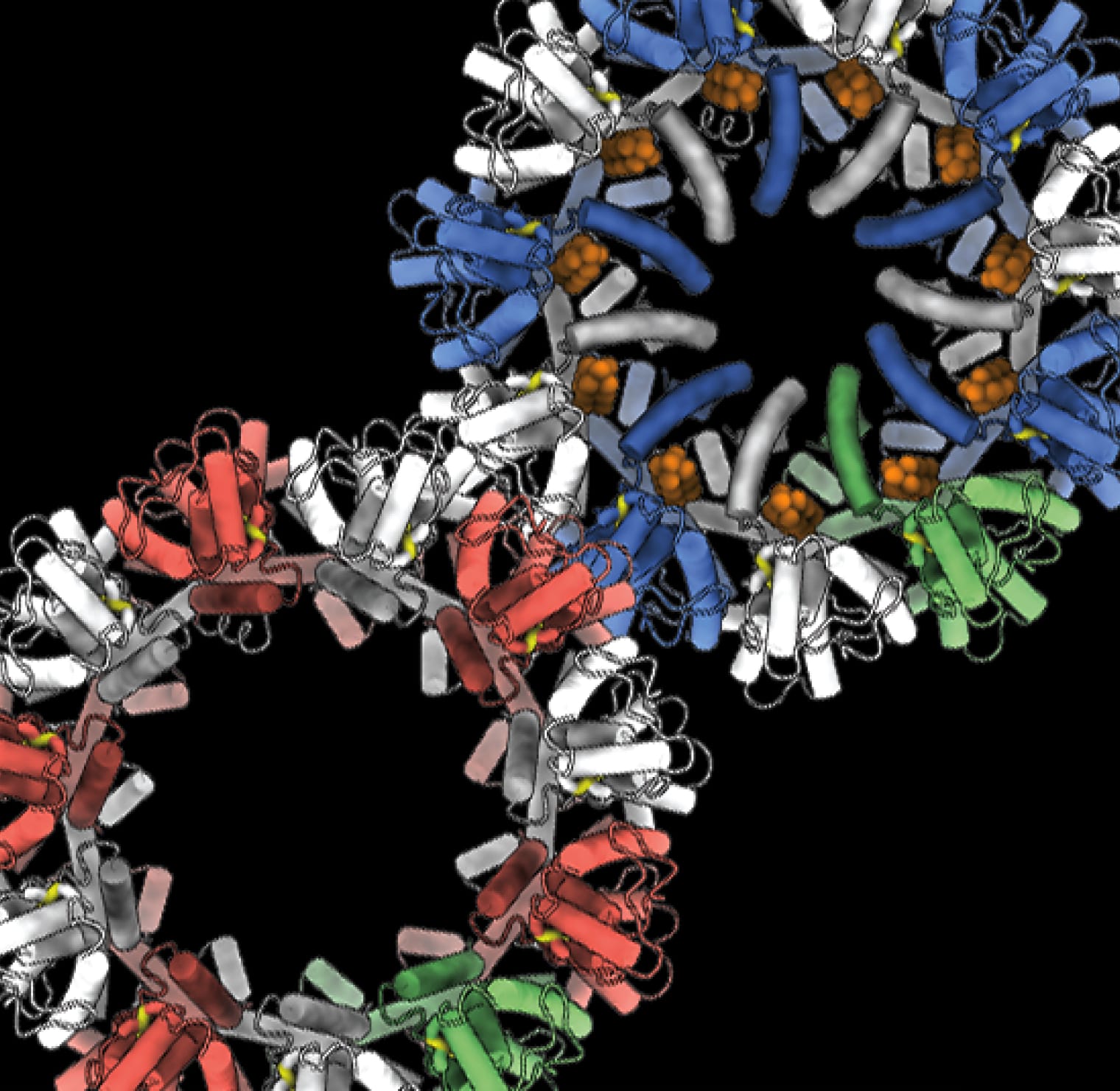
Imagine standing on the moon and having eyes so powerful that you can clearly watch a tennis match on Earth. Now imagine that same visual power packed into a high-tech microscope, and you have cryo-EM — a groundbreaking technology that allows scientists to study the smallest components of life in exquisite detail.
Understanding the shape of these critical molecules is vital for understanding their function in health and disease. Scientists in VAI’s Department of Structural Biology harness cryo-EM and other state-of-the-art techniques to visualize molecules that may serve as treatment targets for cancer, neurological disorders, metabolic diseases, infectious diseases and more. They’re revealing groundbreaking new insights into the most fundamental aspects of biology, from parsing the ways cells sense and respond to the environment to illuminating the intricacies of DNA replication. And they’re laying the foundations for new therapies by revealing how a drug molecule disables its target protein.
Our Faculty

Huilin Li, Ph.D.
Chair and Professor, Department of Structural Biology
Cryo-EM, Structural Biology, DNA Replication and Epigenetics

Juan Du, Ph.D.
Associate Professor, Department of Structural Biology
Structural Biology and Electrophysiology

Wei Lü, Ph.D.
Associate Professor, Department of Structural Biology
Cryo-EM and Structural Biology

Evan Worden, Ph.D.
Assistant Professor, Department of Structural Biology
Structural Biology of Epigenetic Complexes